

Medlink Imaging is a wholly owned subsidiary of Vieworks Co Ltd, a major global supplier of imaging plates, based in South Korea. A key element of the distribution agreement is an initial commitment to acquire US$0.72 million (A$1.0 million) of Rover mobile systems during the first year of the agreement and each year thereafter, once the integration of the detector and software with the Rover is given the green light by regulators.
As part of a development agreement with Vieworks, the current Rover cart and imaging chain will be integrated with Vieworks manufactured digital radiology panels and VXVue software. This Rover Mobile DR with Vieworks panel and software will then be registered with the US Food and Drug Administration (FDA) by Micro-X, with an estimated completion in the December 2022 quarter. Medlink Imaging is the largest master dealer of Vieworks panels in the world, and has received strong interest from its current network of US sub-distributors to add the Rover Mobile DR to its range, with the Rover offering a smaller size, weight and greater manoeuvrability.
Micro-X’s Managing Director Peter Rowland said Vieworks was one of the largest suppliers of detectors worldwide and was well respected internationally.
“This relationship with their US distributor not only positions us well for the US market, but also positions us for further expansion globally,” Mr Rowland said.
Medlink Vice President Michael Farah said the company was looking forward to driving the introduction of the Vieworks Rover.
“Our national network of dealers recognises that the market has called for a robust mobile x-ray product designed to meet and exceed the point of clinical care needs,” Mr Farah said. “The Vieworks Rover will be well received, delivering value and exceptional clinical results.”
Vieworks integration team from Korea arrives at Tonsley next Monday to start the integration with Rover.
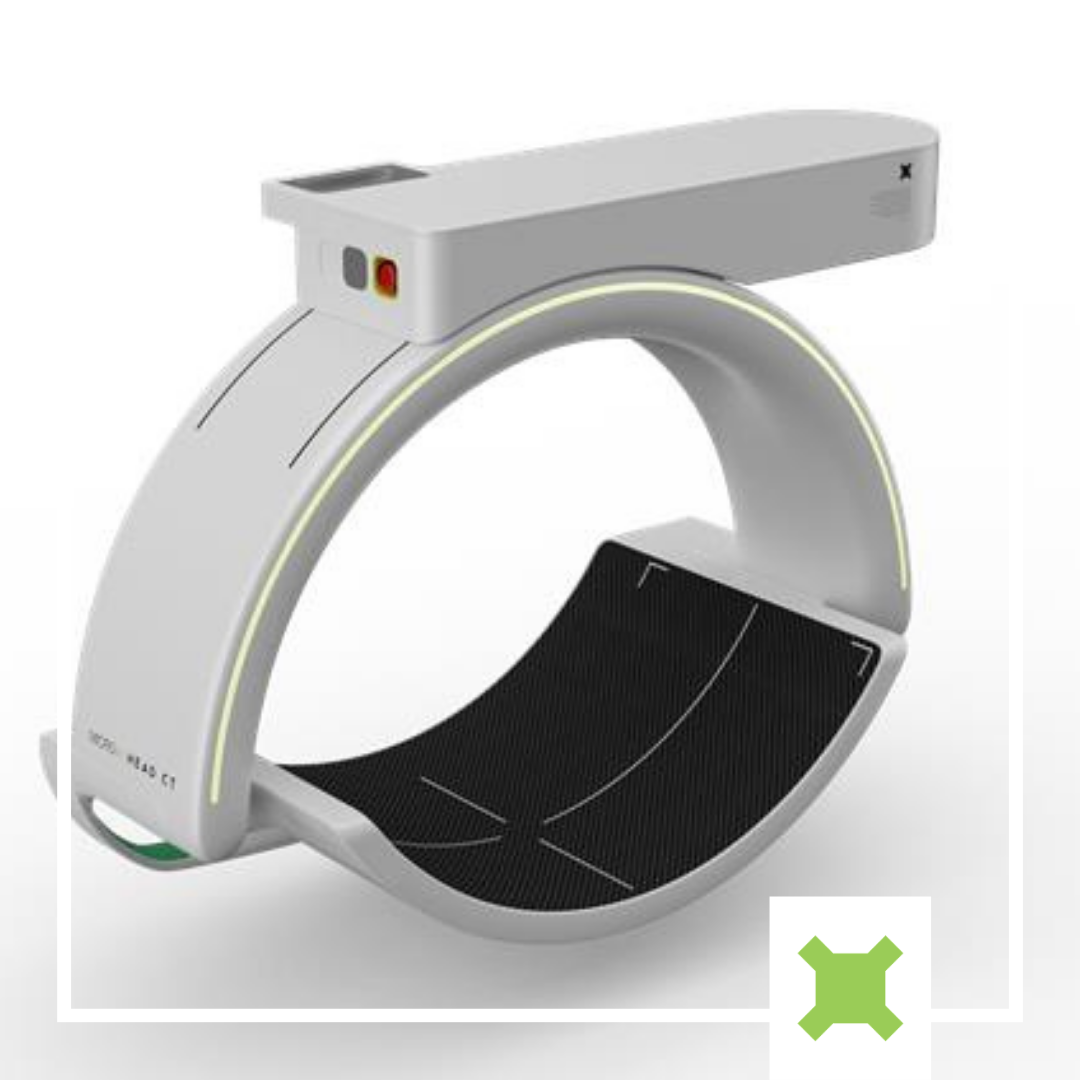

Hear from CEO Kingsley Hall on the latest developments with the Head CT Scanner for stroke


From CT scans in ambulances to virtual hospitals, new Aussie tech and innovations are improving tests, treatment, and are set to bring higher quality care to Australia.


In the final newsletter for 2025, we reflect on some of the achievements of the year, while looking towards what is to come in 2026.

Micro-X creates revolutionary X-ray technology to better lives.
Our Purpose
Find out how Micro-X is creating new opportunities for industries across the world.
Find out more
They’re the visionaries and innovators behind our X-ray technology, products, culture and ethos.
Meet the team